Electric Cell and it’s Working

An electric cell is a device that converts chemical energy into electrical energy through electrochemical reactions.
It typically consists of two electrodes (the anode and cathode) immersed in an electrolyte solution.
The fundamental principle behind its operation is the oxidation-reduction (redox) reaction, which generates a flow of electrons, creating an electric current.
Structure of an Electric Cell:
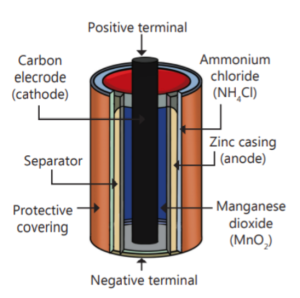
The main components of an electric cell include the electrodes, electrolyte, and a separator.
The anode is the negative terminal where oxidation occurs, releasing electrons. Conversely, the cathode is the positive terminal where reduction takes place, accepting electrons.
The electrolyte is a substance, either liquid or gel, that contains ions and enables the flow of electric charge between the electrodes.
Additionally, a separator serves as a barrier to prevent the electrodes from touching each other, which would lead to a short circuit.
Working of an Electric Cell:
Zn→Zn2++2e−
2MnO2+2e−+2NH4+→Mn2O3+2NH3+H2O
Types of Electric Cells
Electric cells are classified into two main types.
Primary cells are non-rechargeable and rely on irreversible chemical reactions.
Once the energy is depleted, these cells cannot be reused. On the other hand, secondary cells, also known as rechargeable batteries, undergo reversible chemical reactions.
This allows them to be recharged and used multiple times.
How Your Actions Can Impact The Environment
Primary Cells or non rechargeable cells are cheaper single use throwaway goods that cause pollution.
Always consider using rechargeable cells which will also be cost effective in the longer run and less polluting.
For example a single use cell, pack of 4 cost ₹160 and can only be used once.
On the other hand, the rechargeable cell costs ₹500 and can be used for hundreds of charge cycles.
Doing the math, you will recoup the money spent by the 4th charge cycle and you will end up not buying another pack of cells for years / hundreds of charge cycles, thus saving money and the environment.