Understanding How X-Rays Work: A Comprehensive Guide
Are you curious about the inner workings of X-rays? In this article, we delve into the captivating realm of X-ray technology, explaining their fundamental principles and shedding light on their crucial role in various fields.
Join us as we demystify the science behind X-rays and explore their applications.
History Of X-Rays
X-ray technology, a remarkable invention in human history, emerged entirely by chance.
In 1952 VS, Wilhelm Roentgen, a German physicist, stumbled upon this discovery during his experimentation with electron beams in a gas discharge tube.
To his surprise, when the electron beam was activated, a fluorescent screen in his laboratory began to emit a glow.
This finding was unexpected since he had presumed that the surrounding heavy black cardboard would have effectively blocked the majority of the radiation. In the quest for understanding, he conducted further experiments by interposing various objects between the tube and the glowing screen.
To his amazement, even with barriers in place, the screen continued to emit its radiant glow.
However, it was when he fearlessly placed his own hand before the tube that the true marvel of X-rays unfolded.
He witnessed the captivating sight of his bones cast upon the fluorescent screen.
|
|
In that transformative moment, Roentgen not only discovered X-rays themselves but also realized their immense potential in the field of medicine.
This groundbreaking revelation set in motion one of the most significant advancements in medical history.
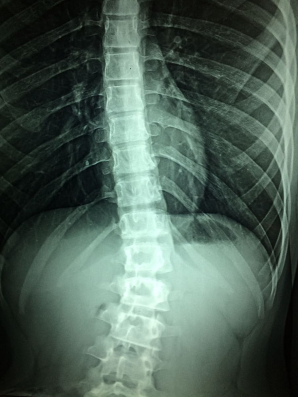
X-ray technology grants doctors the remarkable ability to peer directly through human tissue, effortlessly examining fractures, dental cavities, and even foreign objects swallowed unintentionally.
Beyond this, modified X-ray techniques enable the examination of softer tissues, such as the lungs, blood vessels, and intestines, offering invaluable diagnostic insights with remarkable ease and precision. |
What Are X-Rays?
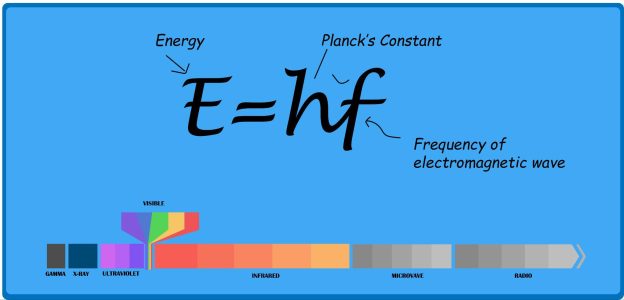
X-rays and visible light rays share a common nature as wavelike forms of electromagnetic energy carried by photons.
However, their distinguishing factor lies in the energy level and wavelength of these photons.
Visible light, perceptible to our eyes, corresponds to a specific wavelength, while X-rays possess higher energy and shorter wavelengths.
On the other end of the spectrum, radio waves have lower energy and longer wavelengths, falling beyond our visual sensitivity.
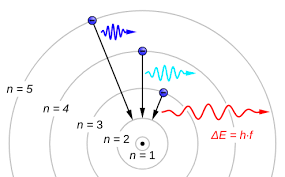
Both visible light and X-rays originate from the movement of electrons within atoms.
Electrons occupy different energy levels or orbitals around the atomic nucleus.
When an electron transitions to a lower orbital, it emits excess energy in the form of a photon, the energy level of which is determined by the extent of the electron’s drop between orbitals.
When a photon interacts with another atom, it may transfer its energy by elevating an electron to a higher orbital.
For this exchange to occur, the energy of the photon must match the energy difference between the electron positions. Otherwise, the photon cannot induce electron orbital transitions.
Visible light photons are effectively absorbed by the atoms constituting our body tissues.
Their energy level aligns with various electron energy differences, facilitating absorption. In contrast, radio waves lack the energy required to cause significant electron orbital shifts and hence pass through most materials.
X-ray photons, possessing excessive energy, also traverse most substances due to a different reason: their energy surpasses the absorption capacity.
Nevertheless, X-ray photons can dislodge electrons from atoms entirely. Upon interaction, some of the X-ray photon’s energy aids in liberating the electron, while the remaining energy propels the electron through space.
Larger atoms exhibit a higher probability of absorbing X-ray photons since their electron orbitals encompass greater energy disparities, better aligning with the photon’s energy level.
In contrast, smaller atoms with narrower energy gaps between electron orbitals are less likely to absorb X-ray photons.
Soft tissues composed of smaller atoms exhibit limited X-ray photon absorption.
However, the calcium atoms comprising bones, being larger in size, possess an enhanced ability to absorb X-ray photons effectively.
In summary, X-rays and visible light rays differ in the energy levels of their photons. While visible light photons are efficiently absorbed by body tissues, X-ray photons, with their higher energy, can pass through most materials while being more readily absorbed by larger atoms such as those found in bones.
The Parts And The Working Of An X-Ray Machine
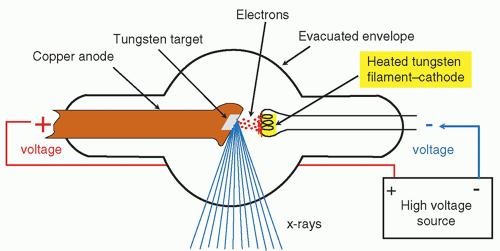
At the core of an X-ray machine lies a vacuum tube housing an electrode pair: a heated cathode and a positively-charged anode.
The cathode, resembling a filament, emits electrons when current passes through it.
Meanwhile, the anode, composed of tungsten, attracts and guides these electrons.
X-ray machines perform seemingly miraculous feats by employing fascinating scientific principles.
They penetrate clothing, flesh, and even metal to reveal the secrets hidden within.
Let’s explore how these machines achieve the remarkable ability to visualize our bones.
With an immense voltage difference between the cathode and anode, electrons accelerate through the tube with tremendous force.
When an electron collides with a tungsten atom, it dislodges an electron from one of the atom’s lower energy levels.
The displaced electron from a higher energy level swiftly transitions to the lower level, releasing surplus energy as an X-ray photon.
Due to the significant energy drop, the resulting photon possesses a high energy level, making it an X-ray photon.
Interestingly, photons can also be generated without colliding with an atom.
An electron racing past an atom may experience sufficient attraction from the atom’s nucleus, causing it to alter its course akin to a comet’s trajectory around the sun.
This deceleration emits excess energy in the form of an X-ray photon.
The intense collisions involved in X-ray production generate substantial heat.
To prevent the anode from melting, a motor rotates it to distribute the electron beam’s impact.
Additionally, a cooling oil bath envelops the mechanism, efficiently dissipating heat.
To confine the X-rays, a robust lead shield encloses the entire setup, preventing their dispersal in all directions.
Only a narrow beam of X-ray photons can escape through a small window in the shield. This beam subsequently traverses a sequence of filters before reaching the patient.
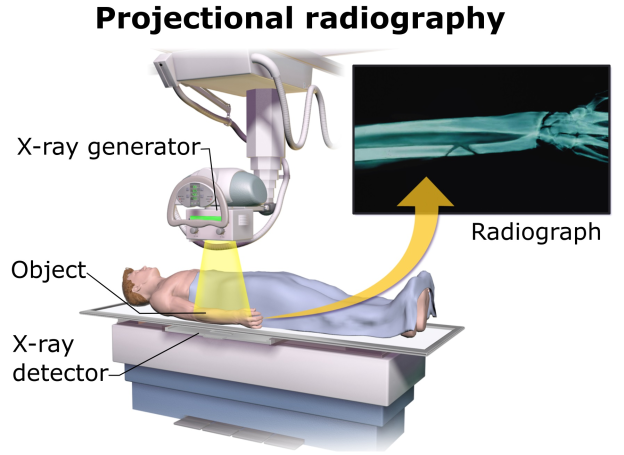
Similar to a conventional camera, the X-ray camera employs film technology, but instead of visible light, it registers the chemical reaction triggered by X-ray photons.
Typically, doctors retain the film image in negative form, wherein areas exposed to more light appear darker, while those exposed to less light appear lighter.
Dense materials like bone exhibit a white appearance, while softer substances appear as shades of gray or black.
By adjusting the X-ray beam’s intensity, doctors can focus on different organs.
In conclusion, X-ray machines harness intricate processes to unravel the hidden mysteries within our bodies. Through their astoundingly precise imaging, medical professionals gain valuable insights into our skeletal structures and beyond.
Are X-Rays Safe?
However, it is important to acknowledge the potential harm associated with X-rays.
In the early stages of X-ray science, doctors and patients were exposed to the beams for extended periods, leading to the development of radiation sickness.
Recognizing this alarming trend, the medical community realized the need for caution.
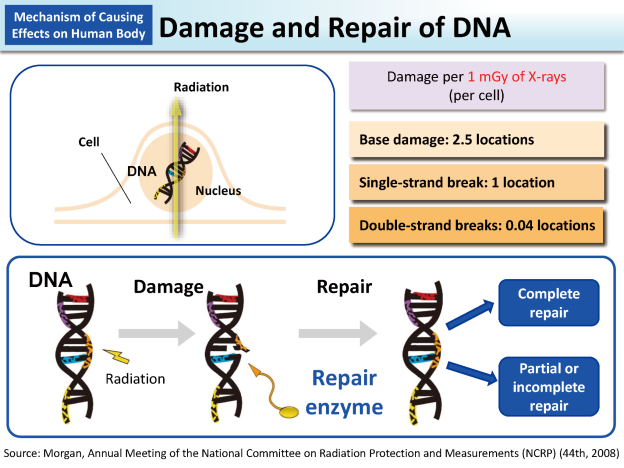
The issue lies in the fact that X-rays belong to the category of ionizing radiation.
Unlike regular light, which has minimal impact on atoms, X-rays have the ability to dislodge electrons from atoms, creating charged particles called ions.
These ions can trigger abnormal chemical reactions within cells, including the disruption of DNA chains.
A cell with a damaged DNA strand may either perish or develop mutations.
Extensive cell death can result in various diseases, while mutated cells may become cancerous and potentially metastasize.
Moreover, mutations occurring in sperm or egg cells can lead to birth defects. Due to these risks, modern medical practices employ X-rays judiciously.
Despite the potential hazards, X-ray scans remain a safer alternative to invasive surgeries. X-ray machines serve as invaluable tools in the field of medicine, enhancing diagnostic capabilities and playing crucial roles in security measures and scientific investigations. Undoubtedly, X-rays rank among the most beneficial inventions in human history.